...And We sent down Iron, in which is great might, as well as many benefits for mankind... (Hadid 57:25)
The above verse in the holy Qur’an uses the Arabic expression "anzalna"which means "sent down" for iron. But why? Early commentators understood this as having a metaphorical meaning to explain that iron has been sent to benefit people. But after understanding the nature of one of the most powerful explosions in the universe, you realize that the direct meaning "being physically sent down from the sky" miraculously points out to a very important scientific fact that was discovered only very recently. To understand and appreciate this miracle of the Qur’an, we will first talk about the life and death of stars and then come back to this verse to describe its relevance in detail.
Just like human beings, stars are also born, live, and die. One big difference is that they can live billions of years compared to the less than 100 years of human life. Also, for us, there is no way of knowing how long we will live or how we will die. But for a star, given its mass, you can predict its lifetime and the way it will die. Stars about the size of our sun live for a long time (a couple of billion years) and die gradually. Whereas massive stars with a mass of about 8 times the mass of our sun or more have short lifetimes (tens of million years) and die in a quick and incredibly violent explosion known as a supernova.
Supernova explosions are one of the most spectacular astronomical events observable by human beings. Normally, in a typical galaxy there are about 10 billion stars. A supernova happens to be one of these ordinary stars until it explodes. During the explosion, the amount of energy released by the supernova can exceed the energy of all the other stars combined in its galaxy! The power of this explosion is far too big even to imagine. The energy released is even greater than the total energy our sun will put out during its 10 billion year life!
The brightest supernova of modern times was an extragalactic supernova recorded in 1987. Since it was the first supernova in 1987, it was labeled as "1987A." This is by far the best studied supernova of all times. In Fig. 1, the left panel shows the region of the sky two weeks after the supernova exploded. The supernova is still very bright. The right panel shows the same region before the explosion, with the arrow indicating the star undergoing the supernova explosion. This particular supernova was 160,000 light years away from us. This means that the actual explosion happened 160,000 years ago, but because it was so far from us, it took 160,000 years for the light rays from the explosion to reach us.
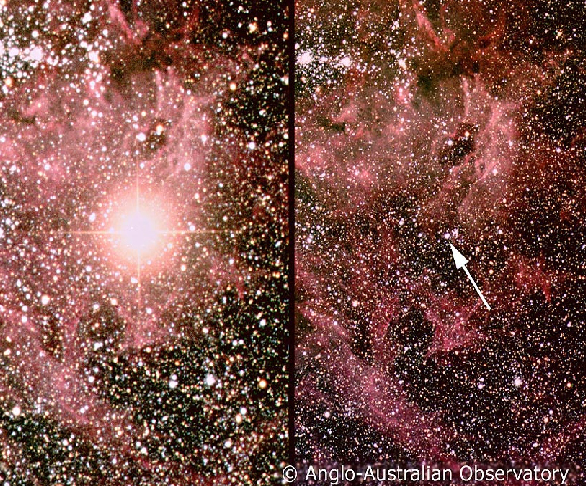 Fig 1. After and before images of the 1987A supernova. The left panel shows the region of the sky two weeks after the 1987A supernova exploded. The supernova is still very bright. The right panel shows the same region before the explosion and the arrow indicates the star undergoing the supernova explosion.
Fig 1. After and before images of the 1987A supernova. The left panel shows the region of the sky two weeks after the 1987A supernova exploded. The supernova is still very bright. The right panel shows the same region before the explosion and the arrow indicates the star undergoing the supernova explosion.
Since the supernova becomes extremely bright, it is even sometimes possible to see it with the naked eye in daytime. In fact, there are historical reports from ancient times concerning supernova explosions. On July 4th, 1054 A.D., Chinese astronomers noticed a "guest star," which was visible in daylight to the naked eye for 23 days. Its remnant was discovered by the British amateur astronomer John Bevis in 1731. We now know that this bright "guest star" was a supernova. Its remnants, known as the Crab Nebula, are shown in the left panel of Fig. 2. This supernova is one of the very few that have been observed in our Milky Way galaxy. The last supernova to explode in our galaxy was in 1607 (see Fig. 2 right panel).
Supernova explosions are one of the most violent events that happen in the universe. They release an unbelievable amount of energy. But why would a star explode anyway? If it has so much energy still, why does it not remain shining peacefully as it does for most of its lifetime? To answer these questions, we need to remember how stars work.
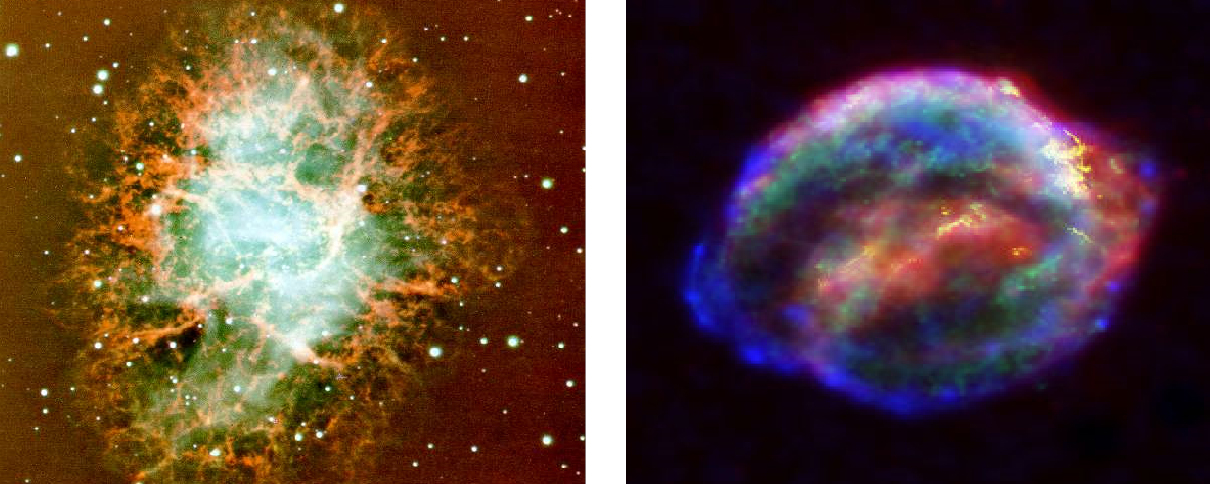 Figure 2 Supernovae remnants. The left panel shows remnant of the supernova that exploded in 1054. It is about 6,500 light years from earth. It consists of diffuse interstellar gas and dust (nebula) spread in a circular region of a diameter of 6 light years.(Image Credit: FORS Team, 8.2-meter VLT, ESO ). The right panel shows remnants of the last supernova, which exploded in our galaxy in 1604. This combined image -- from NASA's Spitzer Space Telescope, Hubble Space Telescope, and e Chandra X-ray Observatory -- unveils a bubble-shaped shroud of gas and dust that is 14 light-years wide and is expanding at 4 million miles per hour (2,000 kilometers per second). It is about 20,000 light years away from us.(Image and caption credit: NASA )/
Figure 2 Supernovae remnants. The left panel shows remnant of the supernova that exploded in 1054. It is about 6,500 light years from earth. It consists of diffuse interstellar gas and dust (nebula) spread in a circular region of a diameter of 6 light years.(Image Credit: FORS Team, 8.2-meter VLT, ESO ). The right panel shows remnants of the last supernova, which exploded in our galaxy in 1604. This combined image -- from NASA's Spitzer Space Telescope, Hubble Space Telescope, and e Chandra X-ray Observatory -- unveils a bubble-shaped shroud of gas and dust that is 14 light-years wide and is expanding at 4 million miles per hour (2,000 kilometers per second). It is about 20,000 light years away from us.(Image and caption credit: NASA )/
Let us start by answering a more basic question: What is the energy source of stars? Stars produce their energy through a process called nuclear fusion. The idea is very simple; you fuse together light nuclei, like hydrogen, to produce heavier nuclei, like helium. In this process, the combined mass of low-mass nuclei is more than the resulting fused massive nucleus. This difference in the masses is converted to energy through the Einstein’s famous E=mc2 equation, where E is the energy released, m is the mass difference that is released in the reaction, and c is the speed of light.
But since the nuclei are positively charged, they repel each other, so there must be extremely high densities and temperatures to overcome this barrier. The most common form of fusion that takes place in stars is the fusion of four hydrogen nuclei to produce one helium nucleus. The temperature needs to be about 8 million C0 for this reaction to occur. It requires higher temperatures to fuse nuclei that are heavier than hydrogen. For example, fusing helium requires temperatures higher than 100 million C0.
During most of their lifetime, stars produce energy by fusing hydrogen into helium. After they run out of hydrogen, if the temperatures in their cores are high enough, they start to fuse helium nuclei into carbon and oxygen. And when they run out of helium, they then start to fuse carbon and oxygen. As mentioned above, fusing heavier elements requires extremely high temperatures and high pressures, so it can only happen for massive stars in the late stages of their lives where such conditions are met. For lighter stars like our sun, temperatures are not enough for this.
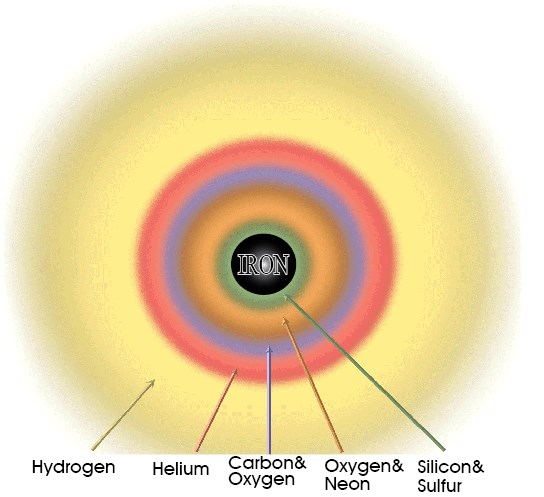 Even for the massive stars, fusion reactions cannot continue forever. The game of building heavier and heavier elements stops when iron is produced in the core. Iron is a very special element. It has the highest binding energy per nucleon. This makes it the most stable element. You actually lose energy when you fuse iron nuclei together rather than gain energy, so elements heavier than iron cannot be made during these cycles. At this stage of its life, the star looks like an onion, in the sense that it has a layered structure. At the core there is iron, surrounding this core there are layers of lighter elements in the order of their atomic weights, with hydrogen being at the outermost layer (see Fig. 3).
Even for the massive stars, fusion reactions cannot continue forever. The game of building heavier and heavier elements stops when iron is produced in the core. Iron is a very special element. It has the highest binding energy per nucleon. This makes it the most stable element. You actually lose energy when you fuse iron nuclei together rather than gain energy, so elements heavier than iron cannot be made during these cycles. At this stage of its life, the star looks like an onion, in the sense that it has a layered structure. At the core there is iron, surrounding this core there are layers of lighter elements in the order of their atomic weights, with hydrogen being at the outermost layer (see Fig. 3).
At this point in time, a very delicate balance that holds the star steady becomes unstable. Normally, the gravitational attraction tries to compress everything together. Therefore, the star has a tendency to collapse onto itself due to gravity. This is balanced by the outward radiation and thermal pressure that are generated by intense fusion reactions that are occurring in the core. But when the core turns into iron, fusion can no longer take place. That means there is no longer a supporting outward force that prevents the star from collapsing.
Figure 3 The onion skin model of a supernova. As it gets close to its death, a pre-supernova star has a layered structure that resembles an onion. Heavy elements produced by nuclear fusion inside the star are concentrated toward the center of the star. Iron, being the most stable element, sits at the core.
After the iron core gets to a certain size, this iron core suddenly collapses onto itself. This collapse happens so fast that it takes only a fraction of a second for the initially earth-sized core to shrink to a radius of 60 km. As the core collapses, the outer layers of the star start to collapse and rush in to fill the gap created by the collapsing core. At this point, another drastic event occurs. The iron core cannot compress forever. When the density in the core reaches the nuclear density, it rebounds. This time the core starts to move outward. But wait, the outer layers are still collapsing! When the collapsing envelope of the star meets with the rebounding core, one of the most powerful explosions in the universe occurs. This is known as a supernova explosion, which can be seen millions of light years away!
This gigantic collision ejects the outer layers of the star into the interstellar medium. As a result of the extreme conditions generated by this, the fusion of heavier elements (heavier even than iron) occurs. As the material from the exploding star collides with the interstellar gas and dust, a whole range of light emissions (from visible to X-ray) occurs. Colorful nebulae (as seen in Fig 2.) that will glow for thousands of years are thus generated.
One of the most important outcomes of supernova explosions is that heavy elements, including iron, are ejected into the interstellar medium. In fact, the only source of heavy elements is such events. All the heavy elements that are found in our solar system are made in one of these violent explosions. They cannot be made in our solar system, as they require extremely high temperatures. That means, the carbon that makes our cells, the hemoglobin that carries oxygen in our blood, and basically almost everything in our body are all made of elements produced in these explosions. We are, in the most literate sense, stardust. It is estimated that on average each carbon atom in our body went through four of these cycles in the past.
It is very clear that these explosions are important for the existence of life on earth. But, understanding the mechanism of these events was only possible in recent years.
It is extremely surprising to hear that iron and almost every other element in our body were made during one of these explosions. Even more astonishing is when we look at what the Holy Qur’an says about iron.
In the Holy Qur’an, there is a special chapter about iron, known as "Hadid" or "Iron". The first thing that surprises you about this chapter is its chapter number: 57. The interesting thing about this is that it matches the atomic weight of one of the isotopes of iron. Iron can have stable isotopes with atomic weights of 54, 56, 57, and 58. The most common form of iron is the one with atomic weight 56 (56Fe).
The reason why this chapter is called "Iron" is the fact that in one verse of this chapter, the Holy Qur’an talks about iron. The second thing that is surprising is the verse number of this particular verse: 25 (or if you count the basmala, it becomes 26). This number (26) is the number of protons in an iron nucleus. And the third numerical code is the total number of verses in this chapter and that is equal to 30. This is equal to the number of neutrons in the most common form of iron nuclei (56Fe). Additional numerical codes can be found through a more detailed inspection of this Qur’anic chapter. No one knew anything about the iron nuclei in the 7th century when the Qur’an was revealed in its present form. And the chance of these numbers being purely coincidental is less than one in a thousand.
After studying these numerical codes, the content of the verse is even more interesting and closely related to our topic. In this verse, the Almighty says: ...And We sent down Iron, in which is great might, as well as many benefits for mankind ... (57:25). The expression "sent down" used for iron in this verse is the English translation of the Arabic word "anzalna". This can either be understood as having a metaphorical meaning to explain that iron was given for the benefit of people. But, if the literal meaning, "being physically sent down from the sky," is considered, we realize that this verse miraculously indicates the scientific fact that all the iron in our solar system came from the sky from supernova explosions.
Another interesting aspect is the fact that the verse says "...in which is great might ..." The Arabic word "shaded" used to describe this can also be translated as "in which is great power" or as "in which is great violence". If you assume this phrase is referring to iron, you can understand it to mean that iron has a great strength. Or a deeper meaning would be to consider the fact that iron nuclei is the most stable nuclei, i.e. the fact that it has the highest binding energy per nucleon. If you consider this phrase as referring to the act of sending down, in that case it reminds you of the great violence in supernova explosions.
In summary, supernova explosions are the violent deaths of massive stars. The course of events that leads to these gigantic explosions, as well as their far reaching consequences, is very interesting. They are the only source of iron and other heavy metals found in our solar system. They show the mercy of God, as life on earth without them would not be possible. At the same time, they can be thought of as an incredible show of divine power. As the Holy Qur’an says at the end of the verse that mentions iron:
Surely God is the All-Strong, the All-Glorious with irresistible might. (Hadid 57:25)
The Anglo-Australian Observatory (http://www.aao.gov.au/)
http://antwrp.gsfc.nasa.gov/apod/ap030914.html
http://www.nasa.gov/multimedia/imagegallery/image_feature_219.html
For more detailed discussion, see for example: Adam Burrows, Nature 403 (6771), 727 (2000).
http://chandra.harvard.edu/resources/illustrations/superPre.html
For example, according to numerological (abjad) calculations, the abjad of the word "Al-Hadeed" in Arabic, when the numerological values of its letters are added up is also 57 and numerological value of the word "Hadid" alone is 26. ( http://www.miraclesofthequran.com/scientific_30.html )